INTRODUCTION
Chronic lymphocytic leukemia (CLL) and SLL represent a single entity in current classifications per the WHO-HAEM5 and ICC. Both are characterized by malignant B cells with an identical immunophenotype, but they are distinguished by the predominant site of disease burden, such as peripheral blood for CLL and nodal compartments for SLL. Most clinical trials and retrospective studies involving CLL/SLL do not separate out SLL patients. In this study, we report reasons for treatment initiation and outcomes in a cohort of SLL patients seen at our institution.
METHODS
Using the Mayo Clinic CLL Database, we retrospectively identified patients with SLL who received first line therapy between 12/1995 and 2/2023. Patients were classified as having SLL if they met at least one of the following criteria for diagnosis: 1) presence of lymphadenopathy or splenomegaly (either by exam or scans) and an absolute clonal B cell count of < 5 x 109/L; or 2) presence of lymphadenopathy or splenomegaly (either by exam or scans) and an absolute lymphocyte count of < 11 x 109/L (if absolute clonal B cell count was not available). Although the presence of anemia (hemoglobin < 11 g/dL) and thrombocytopenia (platelets < 100 x 109/L) are considered as exclusion criteria for a diagnosis of SLL, patients were included if these cytopenia were due to autoimmune hemolytic anemia (AIHA) or immune thrombocytopenia (ITP) with < 50% bone marrow involvement by SLL (bone marrow biopsy confirmed). Patients treated for SLL with extranodal involvement were also included. Indications for SLL treatment were abstracted by chart review and included one of the following: 1) symptomatic or progressive lymphadenopathy and/or splenomegaly, 2) autoimmune cytopenias, 3) extranodal/direct organ infiltration; and 4) other (e.g., cryoglobulinemia, paraneoplastic, non-infiltrative renal disease such as minimal change disease). Overall survival (OS) was defined as the time from treatment start until date of death or last known to be alive. Treatment-free survival (TFS) was defined as the time from treatment start until the earliest of date of next treatment, or death. Kaplan-Meier method was used to display OS and TFS. Cox proportional hazards regression models were used to estimate associations of factors with time-to-event outcomes.
RESULTS
We identified 182 patients with SLL who received first line treatment. The median age at treatment start was 66 years, and 132 (73%) were male. Unmutated IGHV was identified in 60/82 (73%) patients and peripheral blood fluorescent in situ hybridization (FISH) assessed pre-treatment start showed del13q in 10%, trisomy 12 in 43%, del11q in 7%, del17p in 8%, and no abnormalities in 33%. TP53 disruption (del17p and/or TP53 mutation) was present in 7/89 (8%) patients prior to first therapy. The median follow-up after diagnosis was 6.9 years.
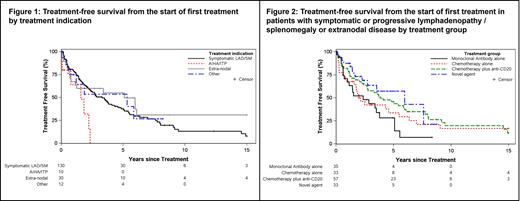
The indications for treatment were grouped as follows: symptomatic or progressive lymphadenopathy/splenomegaly (n=130), extranodal/direct organ infiltration (n=30), AIHA/ITP (n=10), and other (n=12). For the entire cohort, the median TFS was 2.9 years and the median OS was 12.3 years. The median TFS by the treatment indication groups were 3.3, 5.1, 1.6, and 5.3 years, respectively (Figure 1). The median OS by treatment indication groups were 11.1, 10.5, 8.8, and 19.9 years, respectively.
Among the 160 patients with symptomatic or progressive lymphadenopathy/splenomegaly or extranodal disease, the treatments administered include: mAb alone (n=35), chemotherapy alone (n=33), chemoimmunotherapy (n=57), targeted agent (n=33; Bruton tyrosine kinase inhibitor [BTKi] based n=28; venetoclax-based n=3, BTKi + venetoclax n=2), and other (n=2). The median TFS by treatment groups were 2.4, 2.0, 3.8, and 6.0 years (Figure 2), respectively (excluding the small “other” subgroup of 2 patients). The median OS for these groups were 9.5, 9.7, 15.0, and not evaluable, respectively. Only older age at treatment start predicted for shorter TFS (HR 1.02; 95%CI 1.002-1.038; p=0.03) and shorter OS (HR 1.05; 95%CI 1.03-1.07; p<0.01).
CONCLUSIONS
In this study of patients with SLL who received therapy, the most frequent indications for treatment included symptomatic lymphadenopathy and/or splenomegaly, followed by extranodal disease. Trisomy 12 was the most frequently detected abnormality on peripheral blood FISH testing. Treatment with novel agents achieved durable TFS and OS.
Disclosures
Wang:Genentech: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Morphosys: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; BeiGene: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Genmab: Research Funding; Novartis: Research Funding. Kenderian:Morphosys: Research Funding; LEAHLabs: Consultancy, Current equity holder in private company, Research Funding; Mettaforge: Patents & Royalties; Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding, Speakers Bureau; Kite/Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CapstanBio: Consultancy, Other: Scientific advisory board; Torque: Consultancy; Luminary therapeutics: Other: scientific advisory board ; Tolero/Sumtomo: Research Funding; Lentigen: Research Funding; Sendero: Patents & Royalties; MustangBio: Patents & Royalties; Juno/BMS: Other: Membership on an entity's board of directors or advisory committees, Research Funding. Ding:Merck: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; MEI pharama: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Research Funding; AbbVie: Research Funding; DTRM: Research Funding. Muchtar:Protego: Consultancy. Koehler:AbbVie: Consultancy, Other: Advisory Board; Jannsen: Other: Advisory Board; Astra Zeneca: Other: Advisory Board. Tsang:Poseida Therapeutics: Current holder of stock options in a privately-held company. Kay:Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; boehringer ingelheim: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Behring: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Dren Bio: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Agios Pharm: Membership on an entity's Board of Directors or advisory committees; Vincerx: Research Funding; Sunesis: Research Funding; Pharmcyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Bristol Meyer Squib / Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma: Research Funding. Parikh:Bristol Myers Squibb-Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Accerta Pharmaceuticals: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Vincerx: Research Funding; Sunesis: Research Funding; Genentech: Research Funding; Dren Bio: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim Pharmaceuticals Incc: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal